Atomic Structure Links
Electrons and Sublevels Electron Configurations and the Periodic Table Writing Electron Configurations Box and Arrow Configurations using Pauli Exclusion Principle and Hund's Rule Quantum Numbers
|
Box and Arrow Orbital Configurations using Pauli Exclusion Principle and Hund's Rule |
There is yet another way to writing electron configurations. It is called the "Box and Arrow" (or circle and X) orbital configuration.
Sublevels can be broken down into regions called "orbitals". An orbital is defined as the most probable location for finding an electron. Each orbital holds 2 electrons.
| sublevel | # of electrons in each sublevel | # of orbitals | Names of each orbital |
| s | 2 | 1 | s |
| p | 6 | 3 | pz px py |
| d | 10 | 5 | dz2 dxz dyz dxy dx2-y2 |
| f | 14 | 7 | fz3 fxz2 fyz2 fxyz fz(x2-y2) fx(x2-3y2) fy(3x2-y2) |
| g | 18 | 9 |
This sublevel configuration can be broken down into orbitals (boxes).
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6........
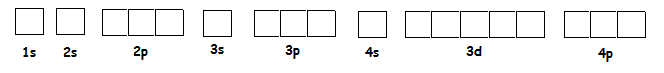
There are a few rules for the box and arrow configurations.
Aufbau Principle - electrons fill orbitals starting at the lowest available energy state before filling higher states (1s before 2s).
Pauli Exclusion Principle
An orbital can hold 0, 1, or 2
electrons only, and if there are two electrons in the
orbital, they must have opposite (paired) spins.
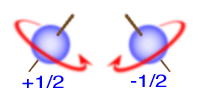
When we draw electrons, we use up and down arrows. So, if an electron is paired up in a box, one arrow is up and the second must be down.
(Therefore, no two electrons in the same atom can have the same set of four Quantum Numbers. )
Hunds Rule
When filling sublevels other than s, electrons are placed in individual orbitals before they are paired up.
Electrons fill like people do on a bus. You would never sit right next to someone you did not know if there are free seats available, unless of course all the seats are taken then you must pair up.
So when working with the p sublevel, electrons fill like this....up, up, up...down, down, down...take a look
on to Quantum Numbers
Electrons and Sublevels Electron Configurations and the Periodic Table Writing Electron Configurations Box and Arrow Configurations using Pauli Exclusion Principle and Hund's Rule Quantum Numbers
back to Atomic Structure Links
![]() Chemical Demonstration Videos
Chemical Demonstration Videos![]()
Pauli Exclusion Principle and Hund's Rule
Box and Arrow Orbital Configurations using. Pauli Exclusion Principle and Hund's Rule
Pauli Exclusion Principle, Aufbau Principle and Hund's Rule
a) violates Hund's Rule, since electrons in 2p are doubled up before each of the orbitals at that energy have one in them b) violates the Aufbau principle, because
Pauli exclusion principle - Wikipedia, the free encyclopedia
The Pauli exclusion principle is the quantum mechanical principle that says that two identical fermions Hund's rule; Fermi hole; Pauli effect; References
Hund's Rule and Pauli Exclusion Principle
Hund's Rule: is when the orbitals contain electrons with equal energy, as they are spinning in the same direction. Also Hund's Rule states that you have to
What are the Pauli Exclusion Principle, Aufbau Principle, and Hunds Rule?
What are the Pauli Exclusion Principle, Aufbau Principle, and Hunds Rule? They are rules we use to fill electron orbital filling diagrams. Fill from the
Hund's rules - Wikipedia, the free encyclopedia
Due to the Pauli exclusion principle, two electrons cannot share the same set of quantum numbers within the same system; therefore, there is room for only two
Aufbau, Pauli, and Hund: The three principals of ...
Aufbau, Pauli, and Hund: The three principals of principles. January 29, 2011 11:15 AM MST ; Report this content; Share this article. Subscribe to author
Difference Between Pauli Exclusion Principle and Hund Rule
What is the difference between Pauli Exclusion Principle and Hund Rule? Pauli Exclusion Principle is about quantum numbers of an atom. Hund rule is
Electronic configuration of atoms using Aufbau, Pauli's principle and Hund's rule - Chemistry
Electronic Configuration of Atoms We know that atomic orbitals are the sub-stationary states or the regions in space where the electrons revolve around the
Three rulesthe aufbau principle, Pauli exclusion ...
Three rulesthe aufbau principle, Pauli exclusion principle, and Hunds ruletell you how to find the electron configurations of atoms. A.
ConversionConversion EmoticonEmoticon